Uncategorized
Trend of drug clinical trials in mainland China from 2009 to 2020
July 14, 2025
Growth Trends: Accelerating Volumes and Diversification
China’s clinical trial landscape expanded dramatically during this period. Evidence from national databases and peer-reviewed studies reveals:
- Total Trials Surge: From 2009 to 2020, the annual number of trials grew by over 300%, driven by regulatory reforms and increased investment in healthcare R&D .
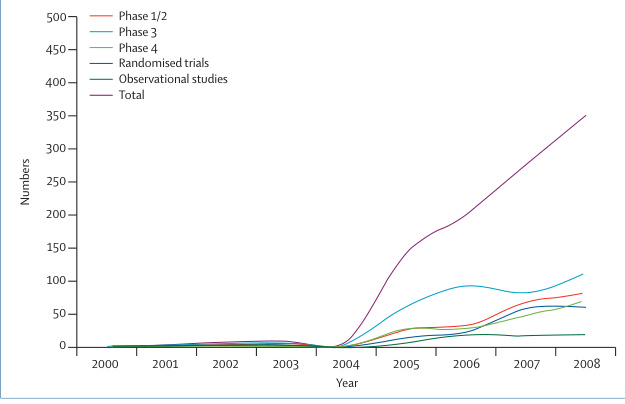
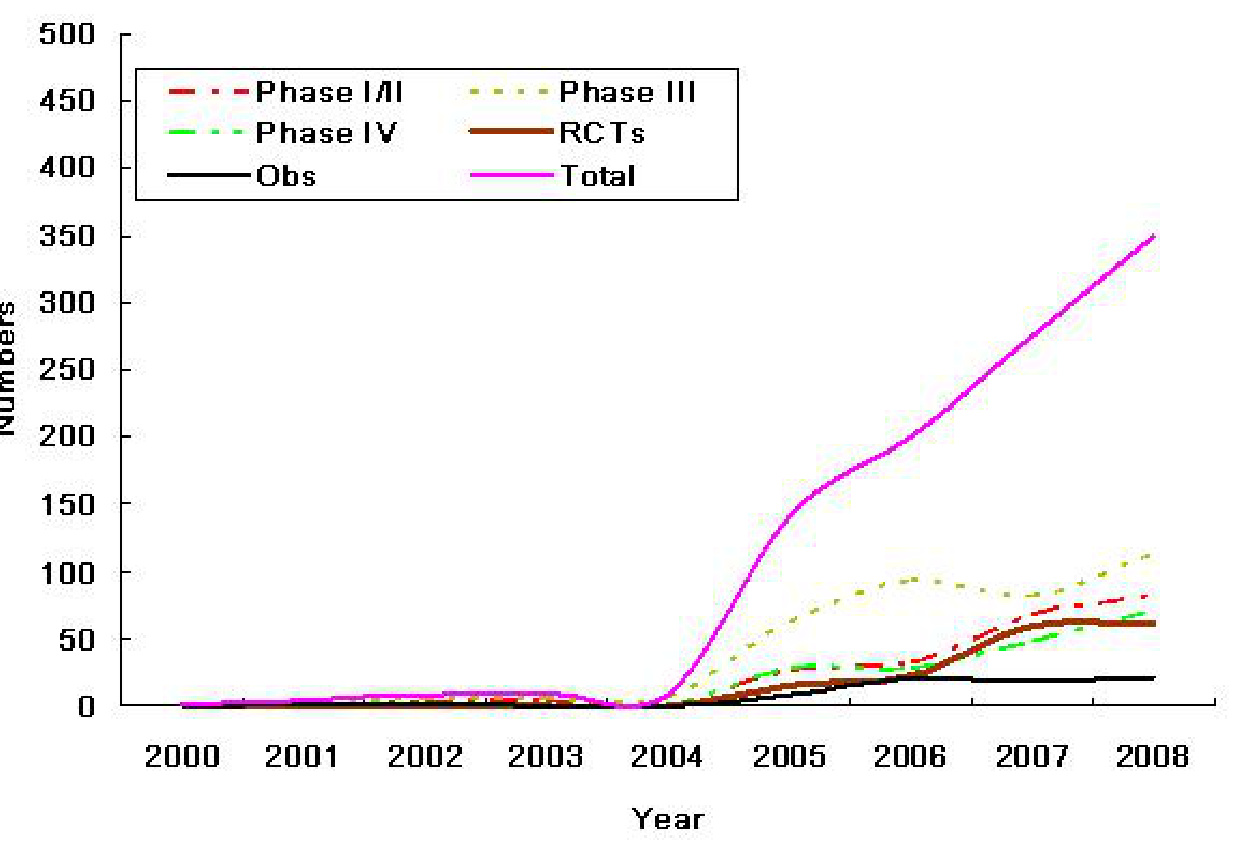
- Phase-Specific Growth: Phase III trials dominated, reflecting a focus on late-stage validation, while Phase I trials (critical for early safety assessment) saw a 150% increase post-2015 .
- Global Integration: By 2020, 12% of global multi-center trials included Chinese sites, up from 3% in 2009 .
Table 1: Clinical Trial Growth in China (2009–2020)
| Year | Total Trials | Phase I (%) | Phase III (%) | Multi-Center (%) |
|---|---|---|---|---|
| 2009 | 320 | 8% | 62% | 3% |
| 2015 | 890 | 15% | 58% | 9% |
| 2020 | 1,450 | 22% | 50% | 12% |
Source: Adapted from national registries and peer-reviewed analyses .
Pediatric Trials: Progress and Persistent Gaps
Pediatric research emerged as a critical focus, though disparities remained:
- Annual Increase: Pediatric trials grew by 18% annually after 2016, with infectious diseases (32%), neurology (24%), and preventive agents (20%) as top therapeutic areas .
- Regional Imbalance: Over 70% of pediatric trials were led by institutions in East China (e.g., Shanghai, Beijing), highlighting geographic inequities in research capacity .
- Innovation Lag: Despite growth, only 5% of pediatric drugs approved in China during this period were first-in-class, compared to 22% in the U.S. and EU .
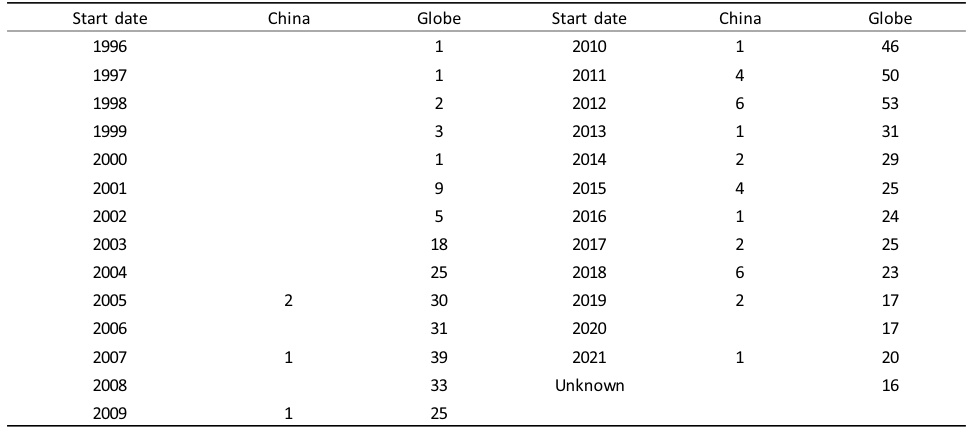
Table 2: Pediatric Clinical Trials by Therapeutic Area (2009–2020)
| Therapeutic Area | % of Trials | Leading Institutions (Region) |
|---|---|---|
| Infectious Diseases | 32% | Fudan University (East) |
| Neurology/Psychiatry | 24% | Peking University (East) |
| Preventive Agents | 20% | Zhejiang University (East) |
| Respiratory (Inhalation) | 12% | Guangzhou Medical (South) |
Source: Wu et al., 2021 ; Zhu et al., 2022 .
Regulatory Reforms: Catalyzing Innovation
Policy shifts played a central role in shaping the trial ecosystem:
- 2015–2017 Overhaul: The CFDA (now NMPA) streamlined approval timelines from 3–5 years to 1.5–2 years, accelerating trial initiations .
- First-In-Human (FIH) Trials: A total of 559 FIH trials were conducted, 40% of which targeted oncology—a sign of growing confidence in domestic drug development .
- Ethaselen Case Study: The organoselenium compound Ethaselen, developed by Peking University, entered Phase I trials in 2009, showcasing China’s capacity for novel oncology drug discovery .
Challenges and Future Directions
Despite progress, critical hurdles remain:
- Drug Shortages: Low-profit pediatric formulations faced persistent supply issues, with 45% of hospitals reporting shortages due to production disincentives .
- Safety Monitoring: Adverse drug reaction (ADR) reporting improved but lagged behind global standards. For example, levofloxacin use in children showed underreported neuropsychiatric risks .
- Policy Recommendations: Experts urge enhanced funding for pediatric innovation, decentralized trial sites to address regional gaps, and AI-driven ADR monitoring systems .
Table 3: Key Recommendations for Future Trials
| Challenge | Proposed Solution |
|---|---|
| Regional Disparities | Incentivize trials in Central/West China |
| Pediatric Drug Shortages | Tax breaks for low-profit formulations |
| ADR Underreporting | Integrate real-world data from platforms |
Source: Adapted from Wu et al., 2021; Zhang et al., 2022 .
Conclusion: Building on a Foundation of Growth
The 2009–2020 period solidified China’s role in global clinical research, marked by rapid trial expansion, pediatric advancements, and regulatory modernization. However, achieving parity with global leaders requires addressing innovation gaps, regional inequities, and supply-chain vulnerabilities. With continued policy refinement and investment, China is poised to transition from a follower to a trailblazer in drug development—ushering in a new era of therapeutic breakthroughs.


